

Welcome to the second instalment in this edu-series: Materials Science 101. The series, split into bite size chunks, will arm you with the basics of materials science so that you can better understand the fast evolving landscape of ‘sustainable’ and next-generation materials.
Materials science encompasses chemistry, physics, biology and engineering and is used to design and build our world – from computer chips to textiles. In part 1, we explained what materials are and how they are developed using materials science. In part 2, we dip our toes into chemistry – metaphorically – to understand how materials are ‘built’ from elements that are made up of atoms, and how those atoms combine to form compounds like cellulose, the fundamental unit of cotton fibres.
What does chemistry tell us about materials?
From part 1 we know that materials are made of ‘matter’. Matter (sometimes simply called ‘stuff’) is anything that contains atoms and takes up space. All materials are created from combinations of atoms. The way these atoms are arranged and how they bond to each other determines how materials look, feel and behave.
Chemistry explains how the atoms bond together in particular arrangements that result in specific material properties. Therefore, understanding basic chemistry is a key facet of understanding the composition and behaviour of materials.
Chemistry 101
If we break down all the matter, or stuff, in the world into its smallest fundamental units (or ingredients, let’s say) we would be left with the elementary particles, or the elements, of which there are 118 (more on that shortly).
Each element contains atoms. Atoms are made of particles called protons and neutrons (huddling in a central ‘nucleus’) and electrons which orbit around them. The particles in atoms determine the chemical properties of the elements.
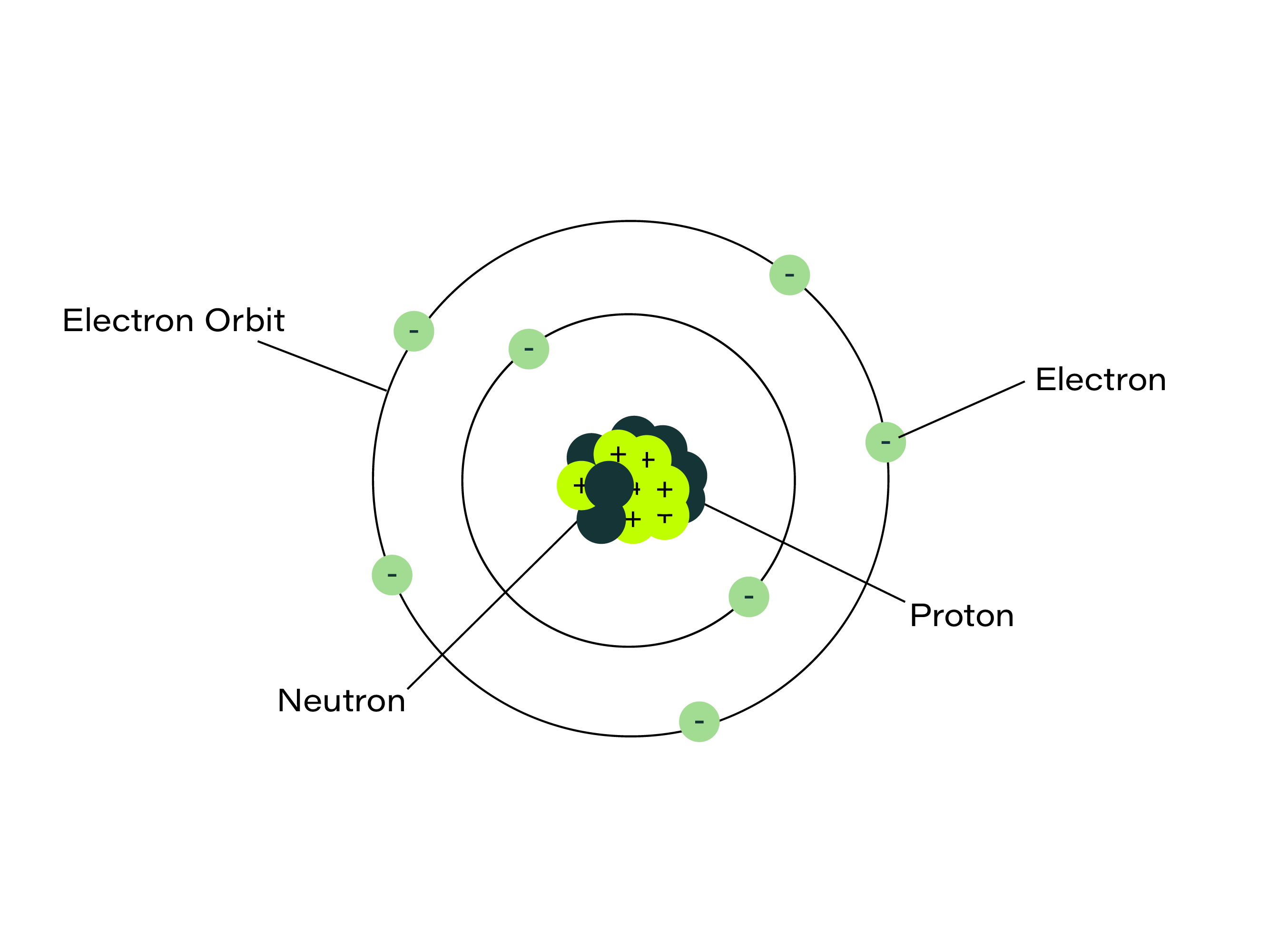
Each element has an ‘atomic number’, which is the number of protons in the nucleus of its atoms. All the 118 elements on planet Earth (our chemical ingredients) are listed on the Periodic Table, and 92 are natural (including hydrogen, copper and carbon) while others are man-made, like technetium.
The element hydrogen sits proudly at number 1 on the table. Its atoms have 1 proton in the nucleus and one electron orbiting the nucleus. Carbon, at position 6, has 6 protons and 6 neutrons in the nucleus, orbited by 6 electrons.
The atomic number is also a handy indicator of how heavy an atom is. Hydrogen (1) and oxygen (8) are both pretty light, taking the form of gases. Copper (29) is heavier, but not as ‘heavy as lead’ (PB), as the saying goes, with 82 protons in its nucleus. Both are solids and both are metals, which have other interesting chemical properties (more on that in later instalments of this series).

Chemistry in context
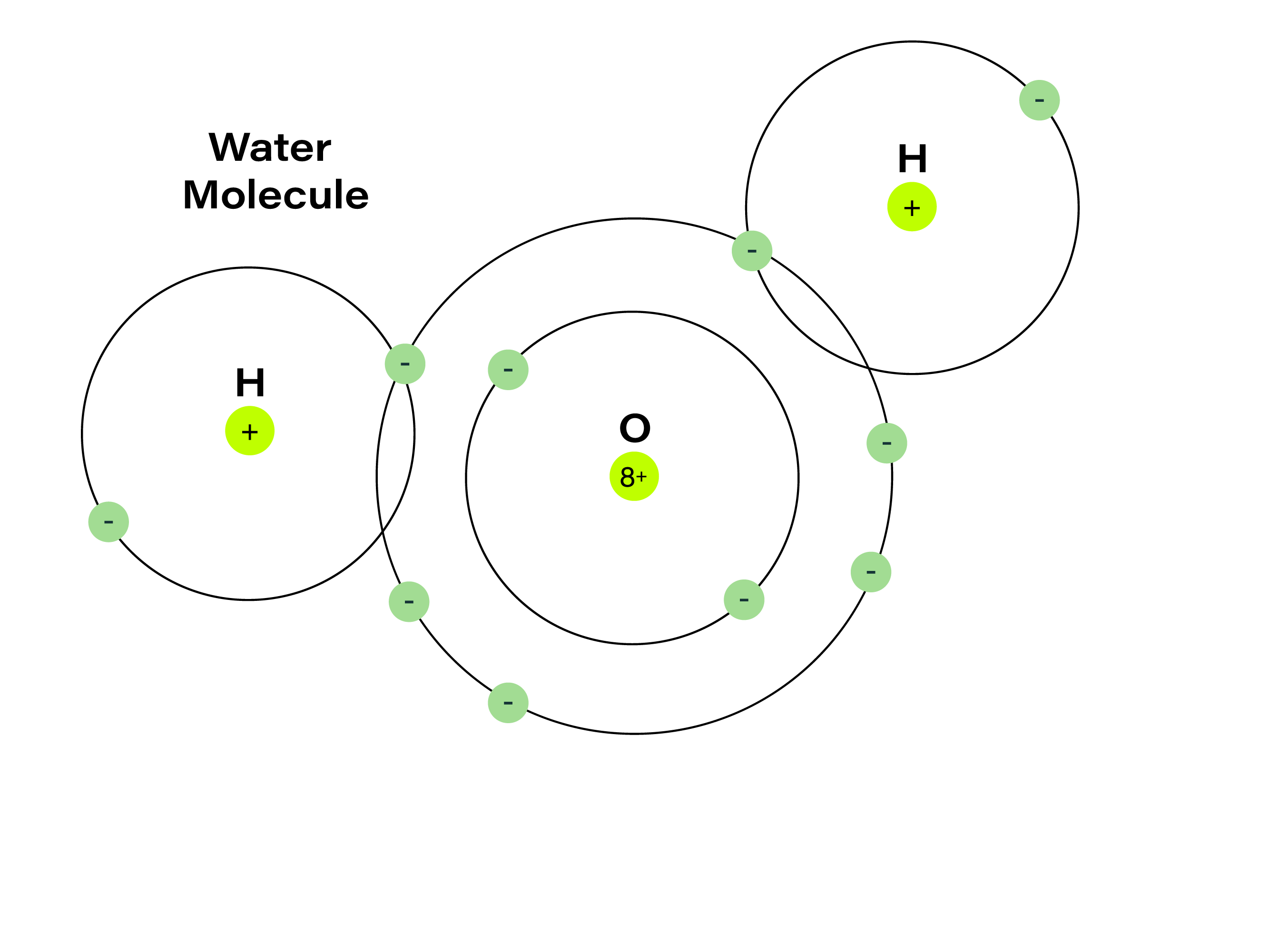
Atoms from the elements can join up to form molecules. Molecules containing atoms from different elements are called compounds (water is a compound of hydrogen and oxygen). But how do atoms from different elements form molecules and how does this influence the characteristics of these molecules?
An example is this: two atoms of the element hydrogen combine with one atom of the element oxygen to make the compound water (H2O). In this example, two gases (hydrogen and oxygen) share electrons to forming a chemical bond. The compound water is a liquid with very different properties than its elements hydrogen and oxygen, which are gases.
Chemical bonds are the result of ‘attraction’ between atoms caused by the sharing and transfer of electrons. These bonds are what allow atoms to form molecules and ultimately materials.
The way atoms bond together determines the resulting properties of molecules and materials. Chemistry, and understanding the inherent properties of atoms and how they form bonds is a foundation for knowing how to manipulate those factors and thereby influence the output (for example, the strength of a material). This is where synthetic chemistry can allow chemists and materials scientists to set up conditions that use the ingredients of nature to create materials with new properties that have been calculated in advance based on the known chemical properties of the ingredients (or elements). One example of this is using fossil fuels mined from the earth to make synthetic plastics.
As mentioned the elements can bond together in a vast array of combinations to form chemical compounds that make all the stuff in our world. Sometimes compounds occur in nature, and sometimes they are made synthetically in labs. As alluded to in the example above, sometimes new compounds are engineered in manufacturing facilities to meet changing material requirements. An example of this would be the extraction of fossil fuels containing naturally occurring compounds of carbon, hydrogen and oxygen and chemically engineering these into new synthetic compounds called polymers, of which polyethylene terephthalate1 (polyester) is one. Polyester was originally developed by a scientist at DuPont as a cheaper and stronger alternative to silk at a time when fibre supplies were low and the demand for strong and inexpensive fibres was rising (particularly for use in war-time textiles).
Chemical properties as related to material properties : natural and synthetic polymers
Cellulose is a chemical compound containing carbon, hydrogen and oxygen. Cellulose is an organic compound, which generally describes any chemical compound2 that contains carbon–hydrogen or carbon-carbon bonds. Cellulose is a naturally occurring compound found in the primary cell wall of many green plants and it is a polymer synthesised in nature, made possible by photosynthesis. Photosynthesis is where plants extract carbon dioxide (CO2) from the atmosphere and ‘metabolise’ it into glucose (C6H12O6). The plant then uses this glucose in a biochemical reaction to synthesise cellulose.
By contrast, polyethylene terephthalate (polyester) is a synthetic polymer. It is synthesised in a lab or manufacturing facility under artificially constructed and controlled conditions. This synthetic process breaks down the molecules containing carbon, hydrogen and oxygen in raw material and synthesises them into ones with specific properties. These unique properties are gained from the new arrangement of the molecules. One of these properties in the final material is higher tensile strength and abrasion resistance than cellulose-based materials like cotton, for example.
Cellulose’s chemical formula is six carbon, ten hydrogen and 5 oxygen atoms bonded together in the molecule (C6H10O5).
Polyester’s chemical formula is (C10H8O4).
This demonstrates that despite both compounds being made up of carbon, hydrogen and oxygen atoms, the number and arrangement of the atoms and the manner in which they bond together results in very different chemical properties. Ultimately, this provides very different material properties that have implications whilst in use, and also at end of life for disposal (as is understood from the difference in biodegradability of naturally occurring polymers like cellulose, compared to that of synthetic ones, like polyester). Furthermore, by understanding the chemical properties of both cellulose and polyester, blended fabrics can been created that take advantage of the beneficial characteristics of both.
Cotton contains mostly cellulose, and when blended with polyester fibres the cotton is more resilient, helping it to maintain its shape in clothing and reducing its likelihood of stretching, wrinkling or staining. The cellulose properties in cotton lend absorbency and can offer a softer and more comfortable feel on the skin compared to polyester.
By creating new molecules and materials, or augmenting existing ones, we can redesign our world using materials that increase performance and reduce the negative impacts on the planet, people and wildlife. This is the power of harnessing chemistry within materials science, but realising this power also relies on skills and knowledge borrowed from biology and physics. It must also be said that the development of synthetic textiles like polyester was a technical leap forward, but has created environmental problems that were unforeseen. Stand by for part 3 in this series where we will explore the role of biology in materials science, and the influence it has on the development of next-generation materials.





